In the fast-transporting environment of modern healthcare, significant care processes depend greatly on the quality of accurate, sterile, and used devices. Among these, the central venous catheter kits play an important role in aggressive processes, especially during intensive care units, emergency, and complex surgery. These kits play an important role in providing safe and efficient venous access to drugs, fluids, and hemodynamic monitoring. As their use becomes rapidly necessary in hospitals, the question of quality becomes paramount. What should hospitals expect in terms of quality standards when selecting the central venous catheter kit?
The Foundation of Patient Safety: High-Quality CVC Kits
The foundation of any safe and successful central venous catheterization lies in the integrity of the CVC kit. From the catheter tube to the introduction needle, guidewire, dilater, and clamp- each component should be manufactured to accurate standards. When the kits fail to meet the standards, the risk multiplies: bloodstream infection, catheter dislocation, bleeding, or vascular trauma. In an era where there has been an increase in probes on infection control in hospitals, choosing a kit that meets both global and regional quality benchmarks is not optional – it is necessary.
What Global Standards Should a CVC Kit Manufacturer in India Follow?
India has emerged as an important player in the global medical equipment market, now exporting to more than 50 countries with many CVC kit manufacturers in India. However, international recognition is possible only when manufacturers align with approved standards such as ISO 13485: 2016, which controls quality management systems for medical devices.
Additionally, CE certification is often expected for distribution in European and other international markets. Hospitals should ensure that the products come from the certified features, where quality control, traceability and safety protocols are non-parasical.
Material Quality and Biocompatibility
The catheter content used in the disposable CVC kit in India should be biocompatible, non-thrombogenic, and curb-resistant. Typically made of polyurethane or silicone, these materials will have to undergo strict tests for compatibility with human tissue. In addition, the sterility of each component is important. Hospitals should verify whether the kits are EO (ethylene oxide), which are sterilized and packed in microbial contamination conditions. Sterilism indicators should also be marked and verified by an independent audit.
Functional Integrity and Ergonomic Design
Quality is not only about material or sterility; It is also about functionality. The guidewire should be glued smoothly, the introductory needles should be sharp for easy puncture, and the clamp should be secured strongly without being slippery. A reliable CVC kit supplier in India should offer ergonomic designs that facilitate ease of insertion and reduce the process time, especially in a high-pressure environment such as the ICU. Color-coded components, minimal handling, and integrated safety features can lead to a longer way to reduce human error.
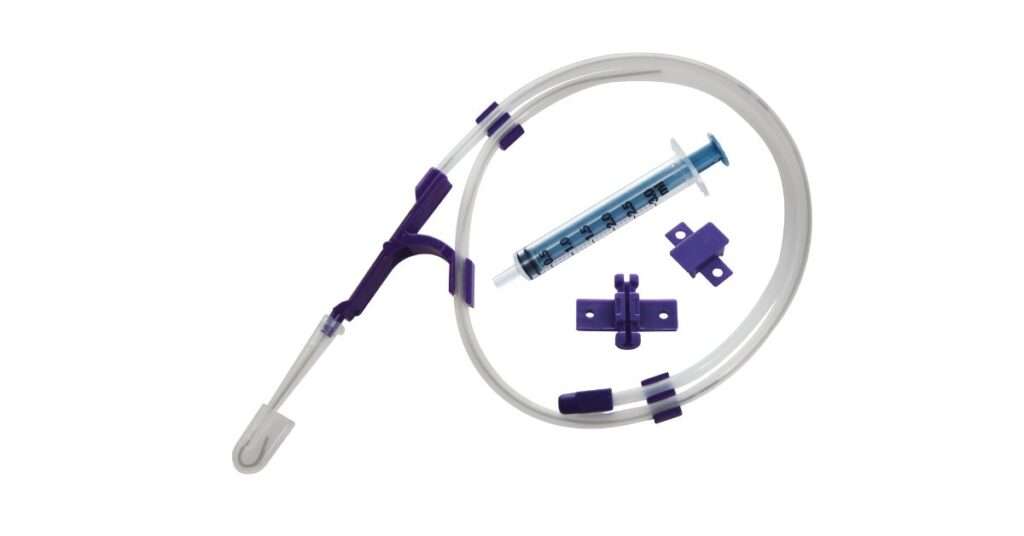
Customization for Clinical Settings
The catheterization care has another emerging standard adaptation. Hospitals are no longer looking for a size-fit-all kit. Instead, they expect CVC kits that are differently adapted to newborns, pediatrics and adult patients.
A reliable manufacturer should provide several lumen options, various catheter lengths, and additional accessories such as determination tools and antimicrobial coatings. When evaluating suppliers, hospitals should inquire about the flexibility offered in the kit configuration.
Batch Traceability and Post-Market Surveillance
A CVC kit is only as reliable as the traceability system supports it. Hospitals should expect manufacturers to maintain a strong batch tracking system that can detect each kit of its production line, date, and even raw materials used. In addition, post-mortem surveillance should be practiced with hard work. Manufacturers must have mechanisms to gather feedback from hospitals, report adverse events, and start a timely recall if necessary.
Packaging Standards and Shelf Life
In a high-length environment, even the packaging of the central venous catheter kit makes a significant difference. The kit should be packed in tamper-evident, sterile packaging that can withstand transport stress. In addition, manufacturers should provide clear labeling, including expiration date, storage instructions, and lot numbers. A high quality kit will maintain its sterile and functional integrity for a specified period for a specified period, often under recommended storage conditions, 2 to 5 years.
Regulatory Transparency and Compliance Documentation
Hospitals should never ignore the importance of regulatory transparency. A prestigious CVC kit in India will provide a certificate of analysis (COA), sterilization report, and material safety data sheet (MSDS) on request. These documents help hospitals to remain in accordance with recognition bodies and ensure that the kits meet the local medical council standards.
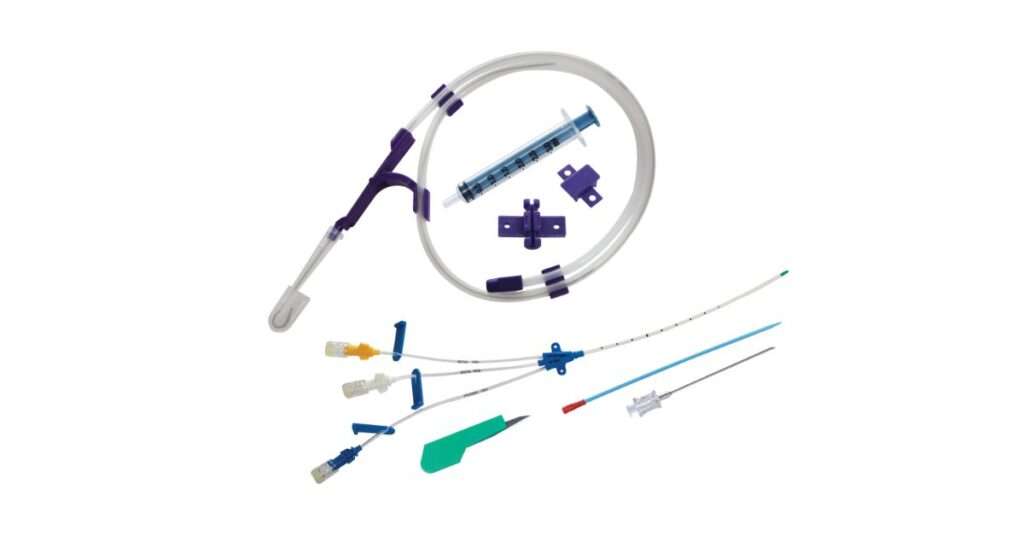
How Prymax Healthcare Upholds These Standards
In this competitive and quality-sensitive environment, Prymax Healthcare has established itself as a reliable name in the field of aggressive medical equipment. As an experienced CVC kit manufacturer in India, Prymax ensures that all its central venous catheters meet the highest standards in quality, safety, and compliance. Each kit is manufactured in ISO-certain features with rigorous quality checks, international material sourcing, and advanced sterilization protocols.
Their range of disposable CVC kits in India includes adapted solutions for various clinical requirements. He is a multi-lumen catheter for intensive care, pediatric lines for newborn use, or antimicrobial options for an infection-prone environment. Prymax also prioritizes traceability, provides detailed documentation, and the product continuously practices post-market quality assessment to guarantee reliability.
Prymax Healthcare empowers hospitals to provide better important care with confidence, by completing the global benchmark and adapting to local clinical needs.
Conclusion
With the safety of the patient on the line, hospitals cannot compromise on the quality of their central venous catheter kit. Every element matters, from international certificates and biocompatible materials to ergonomic design and traceability. A good CVC kit does not serve only one function; It supports a life-saving process.
As the demand for important care processes increases, a partnership with a trusted CVC kit supplier in India becomes a strategic decision. With companies such as Prymax Healthcare, hospitals are assured that they are using kits that not only meet basic standards but also set a new benchmark for excellence.
FAQs – People Also Ask
1. What certifications should a central venous catheter kit have?
Hospitals should look for ISO 13485:2016 and CE certifications to ensure global compliance and product safety.
2. How can hospitals verify the sterility of a disposable CVC kit?
CVC kits should have EO sterilization indicators, batch tracking, and sterilization reports provided by the supplier.
3. Are CVC kits in India suitable for international use?
Yes, many Indian manufacturers like Prymax Healthcare meet international standards and export to over 45 countries.
4. Why is biocompatibility important in central venous catheter kits?
Biocompatible materials reduce the risk of infection, thrombosis, and tissue damage during long-term catheterization.

